
This mnemonic is good for quickly identifying the answer to electronegativity problems, particularly when the answer is more ambiguous such as when elements are not in either the same column or row of the periodic table.Ītomic size trend increases as you go down and to the left on the periodic table.Īs you go to the right, the atomic size trend decreases because you are adding one more proton to the nucleus (the positively-charged center of the atom) each time you move one element to the right. To remember the order of electronegativity of atoms, use the mnemonic “FONClBrISCH.” The atoms in the mnemonic are listed in decreasing order of electronegativity so F>O>N>Cl>Br>I>S>C≈H. The result of this hogging is called induction, which occurs when partial charges appear on atoms as a result of a highly electronegative atom taking electrons. When an electronegative atom like fluorine is next to a less electronegative atom, the more electronegative atom tends to hog or take some of the electrons.

Therefore, fluorine (shown on the periodic table above) is the most electronegative atom on the periodic table. Electronegativity increases as you go to the right and up on the periodic table. If an atom is highly electronegative, it will try to take electrons from its less electronegative neighbors. What is electronegativity? Electronegativity is how much an atom desires electrons. Specifically, we will discuss “what is electronegativity?” and the atomic size trend.
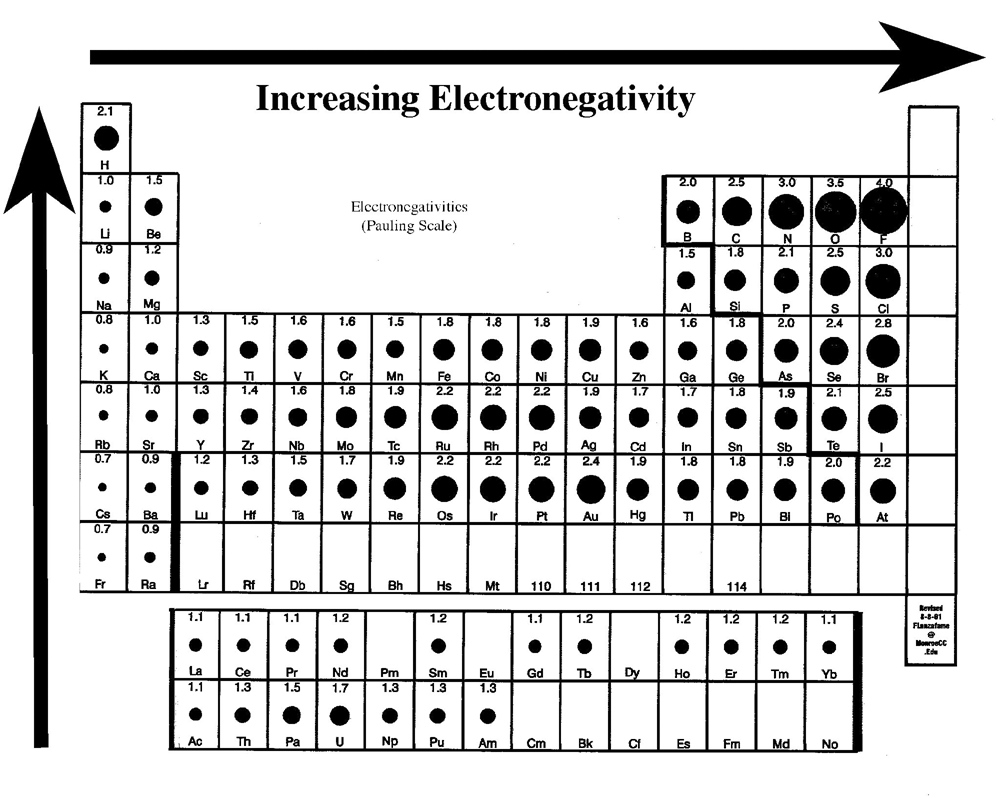
You likely first learned trends of the periodic table in general chemistry, but let’s refresh now. Trends of the periodic table are a foundational concept of organic chemistry.


 0 kommentar(er)
0 kommentar(er)
